Abstract
Introduction: Ibrutinib is approved for all lines of therapy in CLL in the US and the EU. However, front-line ibrutinib studies have largely enrolled older (≥ 65) low-risk patients (Burger, NEJM 2015 n=136, O'Brien ASH 2016 n=31) that might not represent patients seen in clinical practice where treatment patterns and outcomes differ from clinical trials (Mato, Annals Onc 2017). We aimed to study front-line use of ibrutinib in CLL patients treated in the real world focusing on adverse events (AEs), discontinuations, outcomes, and subsequent therapies. To our knowledge, this is the largest series of CLL patients treated with front-line ibrutinib in the real world.
Patients and Methods: We performed a retrospective cohort analysis of CLL patients treated with front-line ibrutinib across 19 US and international academic and community sites. We examined demographics, clinical-molecular-genetic prognostic factors, dosing, discontinuation rate and reasons, AEs, overall response rates (ORR), complete responses (CR), and survival outcomes. We also examined treatment choices following ibrutinib discontinuation, sequencing, and outcomes with subsequent therapies. The primary endpoint was progression-free survival (PFS) measured from the time of ibrutinib initiation until CLL progression, death, or last follow up as estimated by the Kaplan Meier method. Comparisons of outcomes data were made using the log rank (LR) test or Cox regression. Descriptive statisitcs were used for demographic data.
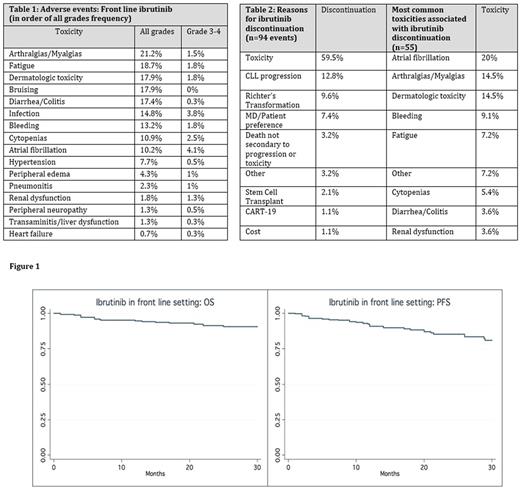
Results: A total of 391 CLL patients were included in this analysis. Median age at ibrutinib initiation was 68 years (range 36-96, 41% age < 65), 62% males, 92% Caucasians, 80% Rai stage ≥2, 30% del17p+, 17% del11q+, 20% TP53 mutation+, 23% complex karyotype (≥ 3 abnormalities), and 67% unmutated IGHV . In all, only 57 patients (14.5%) were genetically low risk (del17p/11q neg, IGHV mutated). Metaphase cytogenetics were concomitantly performed in 76% of patients who underwent FISH testing. For 79 patients with complete data, CLL-IPI risk groups were 2.5% low risk, 20% intermediate risk, 49.5% high risk, and 28% very high risk. Median time from CLL diagnosis to starting ibrutinib was 31 months (0-336). An initial dose < 420 mg daily was administered to 8% of patients and 16% were given ibrutinib in combination (anti-CD20 was most common pair: 95%). During therapy, 17% of patients needed a permanent dose reduction (median continued dose 280 mg) and 42% necessitated dose interruption (median time off ibrutinib 12 days). AE data (all grade, grade ≥ 3) attributed by the treating physician are presented in Table 1. Using modified iwCLL response criteria, ORR was 81.7% (17.4% CR). With a median follow up of 14 months, median PFS and OS have not been reached (PFS and OS at 12 months were 92% and 95% respectively) (Figure 1). In del17p+ patients, PFS and OS at 12 months were 87% and 89% respectively. In univariate analyses including age ≥ 65, sex, del17p+, del11q+, TP53 mutation+, complex karyotype, IGHV status, LDH and β2MG, only del17p+ was associated with inferior PFS (OR 1.91, 95% CI 1.04-3.51, p=.037). Twenty-four percent (n=94) of patients have discontinued ibrutinib after a median time of 6.5 months (0.1-41) (Table 2), and 14% (n=55) have required subsequent therapy. The top 3 regimens following ibrutinib were: anti-CD20 ± chlorambucil 32.6% (ORR= 58%), Venetoclax 18% (ORR= 89%), alternate kinase inhibitor 12.6% (ORR = 40%). FCR / BR was administered to 5 patients. At the time of the analysis the combined ORR following ibrutinib discontinuation on all subsequent therapies was 61% and 69% of patients have not progressed.
Conclusions: In the largest series of front-line ibrutinib-treated CLL patients in the real world, ORR appears comparable to clinical trials, but with different AEs profile. PFS and OS in this high-risk population is comparable to earlier studies, but patients are more likely to have dose-reductions, interruption, and initiate therapy at lower than the FDA-approved dose. Toxicity is the most common reason for discontinuation. Interestingly, many patients did not require immediate treatment following discontinuation suggesting sustained responses. Encouraging responses were observed following ibrutinib discontinuation with no clear standard treatment approach and limited use of CIT in these chemo-naïve patients. This underscores the need for trials studying best strategy post-ibrutinib.
Mato: AbbVie: Consultancy, Research Funding; Janssen: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Research Funding; Pharmacyclics: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy; AstraZeneca: Consultancy; DTRM: Research Funding; Portola: Research Funding. Pagel: Gilead: Consultancy; Pharmacyclics: Consultancy. Brander: Teva Pharmaceuticals, Genentech, AbbVie, Pharmacyclics: Consultancy; Genentech: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cheson: AbbVie, Roche-Genentech, Pharmacyclics, Acerta: Consultancy; Acerta, Pharmacyclics, Epizyme, Gilead, Roche, AbbVi: Other: Institution receives research support . Furman: Abbvie: Consultancy, Honoraria; Genentech: Consultancy; Pharmacyclics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy. Lamanna: Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Tam: Janssen Cilag: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Roche: Honoraria, Research Funding. Lansigan: Spectrum Pharmaceuticals: Consultancy, Research Funding; Seattle Genetics: Consultancy. Barr: Celgene: Consultancy; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Gilead: Consultancy; Seattle Genetics: Consultancy; Infinity: Consultancy. Shadman: Emergent: Research Funding; Acerta Pharma: Research Funding; Pharmacyclics: Other: advisory board, Research Funding; PLEXXIKON: Research Funding; AbbVie: Other: advisory board; Genentech: Consultancy, Research Funding; Celgene: Research Funding; Gilead: Research Funding; Merck: Research Funding; TG Therapeutics: Research Funding. Skarbnik: Novartis: Speakers Bureau; Gilead: Speakers Bureau; Seattle Genetics: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Genentech: Speakers Bureau. Schuster: Seattle Genetics: Consultancy; Gilead: Consultancy, Research Funding; Nordic Nanovector: Consultancy; Merck: Research Funding; Genentech: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Novartis: Consultancy, Research Funding. Shah: Jazz Pharmaceuticals: Consultancy; Oncosec: Equity Ownership; Exelixis: Equity Ownership; Geron: Equity Ownership. Ujjani: Genentech: Consultancy; Abbvie: Research Funding, Speakers Bureau; Gilead: Consultancy; Pharmacyclics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal